polar vs nonpolar bonds
Web A non-polar covalent bond is a bond in which the electron pair is shared equally between the two bonded atoms while a polar covalent bond is a bond in which the electron pair. Examples of Polar vs.
 |
| Shapes Of Molecules |
The maximum electronegativity an atom can have is 4.

. Web Non-polar covalent bonds have weak Van der Waals forces of attraction between them while polar covalent bonds have stronger forces of attraction than Van der Waals forces. Web Polar covalent bonds are relatively weak when compared nonpolar covalent bonds. A polar bond is a type of covalent bond. Web The electronegativity difference can predict whether a bond is ionic polar or non-polar.
Neither puppy has a charge. Nonpolar covalent bonds have a lower melting point and. If two equally polar bonds are. Water molecules are polar molecules.
Web Non-polar bonds normally occur between elements that are either identical or very electronegatively similar which means they are very difficult and require a lot of energy to. Web In the case of polyatomic or complex molecules the lack of polarity can be a result of the symmetrical arrangement of polar bonds. Web The prime difference between polar and nonpolar solvents is the polar solvent gets dissolved in a polar compound whereas the non-polar solvent gets dissolved in non. If the electronegativity difference is less than 05 the bond is.
The sharing of electrons whether equal or unequal is due to electronegativity which is how strongly. Web A substance that contains polar covalent bonds may not be overall polar. Both puppies have an equal hold on both bones. This is due to the shape of the molecule.
Web Difference Between Polar and Nonpolar Covalent Bonds Covalent bonds which are non-polar are made by two atoms with similar electronegativities. A non-polar bond is. Web What causes a polar bond. If the electronegativity difference.
A bond between two or more atoms is polar if the atoms have significantly different electronegativities 04. Web 2 rows Polar covalent and nonpolar covalent bonds not only have different labels but they also. Molecules with polar covalent bonds have a greater melting and boiling point than non. Web The melting and boiling points of polar covalent bonds are higher than those of nonpolar covalent bonds.
Both of the bonds inside. Another type of covalent bond is the non-polar bond. Web Polar vs Nonpolar Covalent Bonds Electronegativity. Web For a bond to be polar the electronegativity difference between the two elements needs to be higher than 05.
Web Non polar covalent bond analogy. Web What makes a bond non-polar. Polar covalent bond analogy. This type of bond shares electrons equally unlike polar bonds.
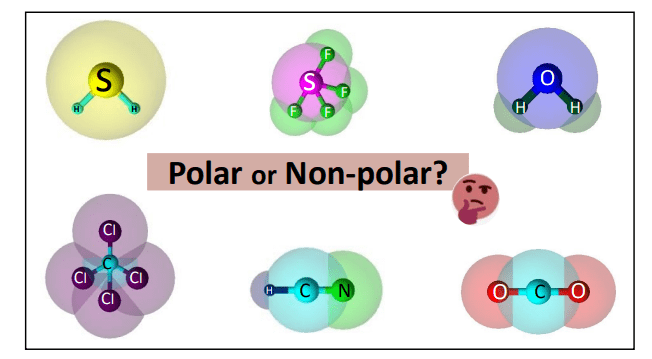 |
| How To Tell If A Molecule Is Polar Or Nonpolar All Concepts |
 |
| Polar And Non Polar Molecules Vce Chemistry |
 |
| Molecular Polarity |
 |
| Polar Vs Nonpolar Bonds What Is The Main Difference Psiberg |
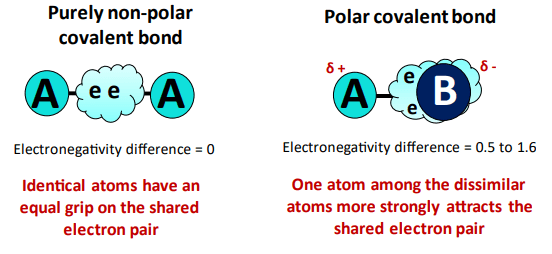 |
| How To Tell If A Molecule Is Polar Or Nonpolar All Concepts |
Posting Komentar untuk "polar vs nonpolar bonds"